Role of Flame Retardants in Plastics
Role of Flame Retardants in Plastics
Polymers can often fuel fires owing to their organic nature. They decompose into combustible products when heated. But, in many fields, polymer usage is limited by their flammability, regardless of their benefits. For example, in electrical, electronic, transportation, construction, etc.
The diffusion of synthetic polymers has greatly increased the:
- fire risk — the probability of fire occurrence and
- fire hazard — the consequence of fire either on humans or on structures.
To fulfill these legal requirements, flame retardants need to be added into the polymer. To increase the escape time of people, the role of these additives is to:
- slow down polymer combustion and degradation (fire extinction)
- reduce smoke emission
- avoid dripping
The severity of the regulations will depend on the time needed to escape an environment.
 Escape Time Without FR (L), Escape Time With FR (R)
Escape Time Without FR (L), Escape Time With FR (R)
Key benefits of flame retardants
-
Prevents fire/retards its growth and spread (flash over)
Under the conditions of fire, the use of flame retardant gives a significant increase in the escape time available.
- It controls the fire properties of combustible items.
- It suppresses fire.
 Flash Over Time vs. Fire Retardant Use
Flash Over Time vs. Fire Retardant Use
-
Protects occupants from the fire effects
The use of fire retardant reduces the flame spread and thus, the rate at which the smoke develops. Less smoke production gives an increase in the escape time available.
- It provides timely notification of the emergency.
- It protects escape routes.
- It provides areas of refuge where necessary and possible.
 Smoke Release vs. Fire Spread
Smoke Release vs. Fire Spread
-
Minimizes the impact of fire
- It provides separation by tenant, occupancy, or maximum area.
- It maintains the structural integrity of the property.
- It provides continued operation of shared properties.
 Example of Functionalities to be Maintained During First Steps of Fire
Example of Functionalities to be Maintained During First Steps of Fire
-
Supports fire service operations
To prevent the fire or retard its growth and spread, material and product performance testing is used. It sets limits on the fire properties of items that represent the major fuels in the system.
- It provides identification of fire location.
- It provides reliable communication with areas of refuge.
- It provides fire department access, control, communication, and selection.
The table below shows a brief overview of the fire retardant and fire resistant characteristics:
| |
Fire Retardant (FR) |
Fire Resistant (FRT) |
| Why |
To save lives |
| How |
Delaying the fire growth |
Limiting the physical progression of fire from one to another area |
| Means |
Decreasing the fire kinetic |
Using fireproof barriers to compartment the fire areas |
| When |
At the early stage of fire, delaying the flash over phenomenon |
During fire from the early to the post flash-over periods |
| What is assessed |
The reaction to fire in terms of contribution to fire:
|
The resistance to fire in terms of maintaining certain functionalities:
- Smoke and heat insulation
- Integrity
- Load bearing
|
| Test scenario
|
- To submit a sample to a heat flux
- To ignite the gaseous decomposition products
- To follow the fire development
|
- To submit the sample to an increasing heat flux
- To follow the functionality evolution during the exposure time
|
| Key parameters
|
- Heat release
- Dripping
- Flame spread
- Smoke opacity
- Smoke toxicity
|
Time failure of functionality studied:
- Smoke insulation
- Heat insulation
- Integrity
- Load bearing
|
Assessing fire safety criteria
One key criterion to consider for the development of FR foam is the standard the material must pass.
The majority of fire safety requirements consist of material fire performance test criteria. This measures how well the FR retards the growth and spread of fire. The test methods are generally based on the measurement of the flame-spread speed. The regulations depend on the region/country, ignition source, and final application.
The severity of the test depends on the specific environment in which the material is used. In general, the higher the severity of the test the higher the concentration of phosphorus FR required to pass the test.
Flame Retardants: How do they work?
Flame Retardants: How do they work?
 Fire is the result of three factors:
Fire is the result of three factors:
Heat produces flammable gases from the pyrolysis of polymer. Then, an adequate ratio between these gases and oxygen leads to the ignition of the polymer. The combustion leads to the production of heat that is spread out (delta H1) and fed back (delta H2). The heat feedback pyrolyzes the polymer and keeps the combustion going.
To limit the establishment of this combustion circle, one or several ingredients have to be removed. Several techniques are available in order to break down this combustion circle. Flame retardants have to inhibit or even suppress the combustion process.
Depending on the polymer and the fire safety test, flame retardants interfere in one or several stages of the combustion process, like:
- heating
- decomposition
- ignition
- flame spread
- smoke process
Flame retardants can act chemically in the condensed/gas phase, and/or physically. However, we have to remember that both of them occur during a complex process with many simultaneous reactions. Let's understand these mechanisms in detail.
The effect of chemical reactions
Condensed phase reactions
In the condensed phase two types of reactions can take place:
- Breakdown of the polymer can be accelerated by flame retardants. It leads to a pronounced flow of the polymer which decreases the impact of the flame which breaks away.
- Flame retardants can cause a layer of carbon (charring) on the polymer's surface. This occurs, for example, by the dehydrating action of the flame retardant. This generates double bonds in the polymer. These processes form a carbonaceous layer by cyclizing and cross-linking processes.
 Char and Intumescence Formation
Char and Intumescence Formation
Intumescence: A unique condensed phase flame retardant mechanism
Flame retardation by intumescence is a special case of a condensed phase mechanism. The activity in this case occurs in the condensed phase. The radical trap mechanism in the gaseous phase is not involved.
In intumescence, less amount of fuel is produced. Here, char is formed instead of combustible gases. The intumescent char has a special active role in the process. It constitutes a two-way barrier:
- the hindering of the passage of the combustible gases and molten polymer to the flame and
- the shielding of the polymer from the heat of the flame.
A considerable number of intumescent systems have been developed in the last 15 years. They all seem to be based on the application of 3 basic ingredients:
- A catalyst (acid source)
- A charring agent
- A blowing agent (spumific)
Additives combining the above three ingredients leading to an intumescent effect are commercially available. However, intumescent formulations can be developed and are more suitable than some commercial grades for specific applications. The table below summarizes the usual catalyst, charring, and blowing agents.
|
Catalyst (Acid Source)
|
Charring Agents |
Blowing Agents (Spumific)
|
| Ammonium salts phosphates, polyphosphates |
Polyhydric compounds |
Amines/amides |
|
|
- Starch
- Dextrin
- Sorbitol pentaerythritol, monomer, dimer, trimer
- Phenol-formaldehyde resins
- Methylol melamine
|
- Urea
- Urea-formaldehyde resins
- Dicyandiamide
- Melamine
- Polyamides
|
| Phosphates of amine or amide |
Others charring |
- |
- Products of reaction of urea or guanidyl urea with phosphoric acids
- Melamine phosphate
- Product of reaction of ammonia with P2O5
|
Polymers (polyurethane, polyamide) |
- |
- Organophosphorus compounds
- Tricresyl phosphate
|
- |
- |
- Alkyl phosphates
- Haloalkyl phosphates
|
- |
- |
Gas phase reactions
The flame retardant or its degradation products stop the radical mechanism of the combustion process. It takes place in the gas phase. The exothermic processes, which occur in the flame, are thus stopped. The system cools down and the supply of flammable gases is reduced and eventually completely suppressed.
The high-reactive radicals HO· and H· can react in the gas phase with other radicals. For example, halogenated radicals X· resulted from flame retardant degradation. Less reactive radicals which decrease the kinetics of the combustion are created (see figure below).
Flame inhibition studies have shown that the effectiveness decreases in the order: HI>HBr>HCl>HF
 Mechanism of Action of Halogenated Flame Retardants
Mechanism of Action of Halogenated Flame Retardants
The effectiveness of fluorides is too low and the iodides are thermally unstable at processing temperature. Thus, brominated compounds and chlorinated organic compounds are generally used. The choice depends on the polymer type. The essential criteria to be considered are:
- the behavior of the halogenated FR in processing conditions. For example, stability, melting, distribution, etc.
- the effect on properties and long-term stability of the resulting material.
Moreover, it is particularly recommended to use an additive that produces halide to the flame at the same range of temperature as that of polymer degradation into combustible volatile products. Then the fuel and inhibitor would reach the gas phase according to the "right place at the right time" principle. The most effective FR polymeric materials are as follows:
- Halogen-based polymers — E.g., polyvinyl chloride (PVC), chlorinated polyvinyl chloride (CPVC), fluorinated ethylene propylene (FEP), polyvinylidene fluoride (PVDF), etc.
- Additives — E.g., cellulose propionate (CP), tetrabromobisphenol A (TBBA), decabromodiphenyl ether (DECA), etc.
However, the improvement of fire performance depends on the type of fire tests i.e., the application. They illustrate the previously described chemical modes of action. Severe perturbations of the kinetic mechanism of the combustion lead to incomplete combustion.
Synergism with Sb2O3
To be efficient the trapping free radicals need to reach the flame in the gas phase. The addition of antimony trioxide allows the formation of volatile antimony species. These are capable of interrupting the combustion process. For example, antimony halides or antimonyoxyhalides. They do so by inhibiting H* radicals by a series of reactions proposed below.
This phenomenon explains the synergistic effect between halogenated compounds and antimony trioxide (Sb2O3). For most applications, these two ingredients are present in the formulations.
 Synergistic Effect Between Halogenated Compounds and Antimony Trioxide (Sb2O3)
Synergistic Effect Between Halogenated Compounds and Antimony Trioxide (Sb2O3)
The effect of physical mechanisms
Formation of a protective layer
The additives can form a shield with low thermal conductivity, through an external heat flux. This can reduce the heat transfer delta H2 (from the heat source to the material). It then reduces the degradation rate of the polymer and decreases the "fuel flow" (pyrolysis gases from the degradation of the material). This feeds the flame.
Phosphorus additives may act the same way. Their pyrolysis leads to thermally stable pyro- or polyphosphoric compounds. This forms a protective vitreous barrier. The same mechanism can be observed using boric acid-based additives, zinc borates, or low-melting glasses.
 Formation of Protective Layer Inhibiting Combustion and Volatiles
Formation of Protective Layer Inhibiting Combustion and Volatiles
Cooling effect
The degradation reactions of the additive can influence the energy balance of combustion. The additive can degrade endothermally. This cools the substrate to a temperature that is below the one required for sustaining the combustion process. Different metal hydroxides follow this principle. Its efficiency depends on the amount incorporated in the polymer.
Fluid dilution effect
The dilution of the fuel in the solid and gaseous phases takes place by the incorporation of:
- inert substances like fillers, for e.g., talc or chalk and
- additives that evolve as inert gases on decomposition.
As a result, the lower ignition limit of the gas mixture is not reached. Recent works have shown the isolating effect of a high amount of ash (resulting from certain silica-based fillers) in fire-retarded systems. Moreover, it also highlights the opposite effect as the thermal degradation of the polymer in the bulk is increased. This takes place by the heat conductivity of the filled material.
Different flame retardants have varying mechanisms of action. They can affect the properties of the plastic material in many ways. Understanding the mechanisms of flame retardants is supercritical. It helps you select the appropriate flame retardant without compromising on properties. Let's have a look at the types of flame retardants to help you make informed decisions.
Types of Flame Retardants
Types of Flame Retardants
There are several chemical classes of flame retardants used with polymers, such as:
Apart from these chemical classes, there are other flame retardants that can be incorporated into a polymer. They may act as additive and reactive flame retardants. Both categories may influence similar properties of different polymers. These include viscosity, flexibility, density, etc. Some characteristics of additive and reactive flame retardants are mentioned in the table below.
| Additive Flame Retardants |
Reactive Flame Retardants |
| Added to the polymer through physical mixing |
Added to the polymer by chemical reactions |
| Do not bind to the polymer chemically (do not undergo any chemical reactions) |
Once incorporated become a permanent part of the polymeric structure (bind chemically) |
| Can be incorporated into the polymeric mixture at any stage of its manufacturing and hence have an added advantage over the reactive FRs |
Must be incorporated only during the early stages of manufacturing |
Brominated flame retardants
Brominated flame retardants (BFRs) are by far the most commonly used class of FRs today. This family of flame retardants is very versatile. It provides the best balance between flame retardant performance, mechanical properties, processability, and cost in use.
Brominated FRs for industrial use are produced by the bromination of Bisphenol-A with bromine. This takes place in the presence of a solvent, such as:
- methanol or a halocarbon
- 50% hydro-bromic acid
- aqueous alkyl monoethers
BFRs when combined with minerals help in improving the mechanical properties. They reduce the opacity and corrosivity of the fumes generated. This helps to diminish the environmental hazards arising from the incineration of fumes. They can provide efficient solutions to meet the regulation requirements. They also offer outstanding performances to the products.
Discover brominated flame retardants available in our database that show the following features.
Chlorinated flame retardants
Chlorinated compounds are molecules containing high concentrations of chlorine. They act chemically in the gas phase. They are often used in combination with antimony trioxide as a synergist. Chlorinated compounds can be distinguished into two main components:
- Chlorinated paraffin
- Chlorinated alkyl phosphate
There are several parameters to consider while selecting a chlorinated compound. These include the chlorine content, thermal stability, volatility, and physical form.
Chlorinated paraffins
The general structure of chlorinated resin is:
There are various products available depending on the length of the paraffinic chain. Liquid grades are produced from short-chain paraffins. Whereas, the solid grades, containing 70-72% of chlorine, are produced from higher molecular waxes.
The key application of chlorinated resins is as a plasticizer for flexible PVC in combination with DOP or DINP. This resin improves flame retardant properties in applications like flooring and cables. Solid grades with high chlorine content as used in thermoplastics. For example, LDPE in CTI cable jacketing in combination with antimony trioxide.
Chlorinated alkyl phosphates
The most common molecules are:
TCEP Tris(2-Chloroethyl)phosphate (L); TCPP Tris (2-Chloro-1-methylethyl)phosphate (C); TDPP Tris (2-Chloro-1-(chloromethyl)ethyl)phosphate (R)
The main application of these products is in rigid and flexible polyurethane foam. They are generally introduced at a concentration between 5 and 15% depending on foam density and test severity. Examples of flame retardant standards achievable with chlorinated phosphorus products are:
- Flexible foam BS4735
- Rigid foam BS 476, NFP92-501, DIN 4102
Chlorinated cycloaliphatics
 Dodecachlorodimethanodibenzocyclo-octane is a commercially available molecule. Its key benefits are:
Dodecachlorodimethanodibenzocyclo-octane is a commercially available molecule. Its key benefits are:
- High-temperature resistance up to 320°C
- Good resistance to UV aging
- Non-plasticizing product
- Insoluble filler and non-blooming
- CTI values greater than 400°C in FR nylon
- Low smoke generation
- Low-density and cost-effective
This product can be used in numerous polymers including polyamide, polyolefins, and polypropylene. They can be combined with various synergists like antimony trioxide and zinc borate.
Discover chlorinated flame retardants available in our database that show the following features.
Organophosphorus flame retardants
One of the major classes of flame retardants for thermoplastics and polyurethane foams is organic phosphorus compounds. These are typically phosphates and phosphonates. They may also include phosphorus-halogen compounds and blends of phosphorus with halogenated FRs (brominated FRs). Thermoplastic alloys such as PC/ABS and PPO/HIPS are often required to meet stringent FR standards such as UL94 V0.
Phosphate-based FRs work efficiently in these resins. They have good physical properties and UV stability. In many applications, rigid and flexible PU foams are required to exhibit a degree of flammability resistance. This enables it to pass specific flammability tests in any given country. Phosphorus-based flame retardants, both chlorinated (chlorophosphates) and non-halogenated are extensively used in these applications. They are considered an ideal choice having a good balance of:
- processability
- flame retardancy, and
- physical properties
In some instances, phosphorus bromine blends are used particularly where low scorch is required.
PUR foam producers can choose between reactive additive, halogenated, and non-halogenated phosphorus-based FRs. This depends on the final application, key requirements, and the flammability standards they must meet. These options provide a versatile selection for addressing the market needs of:
- performance
- compatibility
- efficiency
- physical properties
- processability
- cost
There are several phosphate-based molecules available in the market for flame retardancy. Some common products based on phosphate molecules are:
Triphenylphosphate (TPP), Tricresylphosphate (TCP), Cresyldiphenyl phosphate
(CDP), Tri(isopropylphenyl)phosphate (TIPP) [From Left to Right]
Triphenyl phosphate — It can be used in ABS/PC blends, in other engineering plastics like PPO, and eventually in phenolic resins.
Tricresyl phosphate — It is mainly used in PVC as a flame retardant plasticizer and in styrenic compositions. Commercially available products are a mixture of ortho, meta, and para isomers. However, the ortho is very toxic and excluded as much as possible.
Bis-arylphosphates
Commercial bis-arylphosphates are resorcinol bis-diphenyl phosphate (RDP) and bisphenol A bis-diphenyl phosphate (BDP).
Resorcinol bis diphenylphosphate (RDP)
Bisphenol A bis-diphenylphosphate (BDP)
RDP — It is a colorless liquid generally used in ABS/PC, PBT, and PPO. These products exhibit lower volatility, high thermal resistance, and lower plasticizing effects compared to arylphosphates or alkylphosphates. 10-15 phr are generally needed to pass the traditional FR test. At lower levels, RDP can improve the processability in thin wall injection molding of ABS and styrenics.
BDP — It is similar to RDP and it is used in the same applications at around 20phr. Compared to RDP, BDP provides better melt stability to polymers and lower volatility. The product also has good hydrolytic stability beneficial for polymers like polycarbonate.
Alkyl phosphonates
The general structure of a phosphonate is shown below:
 Dimethyl methyl phosphonate is a very effective flame retardant due to its high phosphorus content. However, the high volatility limits its use in rigid PU and highly filled polyester.
Dimethyl methyl phosphonate is a very effective flame retardant due to its high phosphorus content. However, the high volatility limits its use in rigid PU and highly filled polyester.
Dimeric or oligomeric cyclic phosphates
They are generally highly viscous liquids and, thus, are difficult to handle. Some producers are proposing masterbatch solutions. Dimeric cyclic phosphonate can be introduced in the PET at around 6 wt% for FR PET fibers. It can be used in rigid polyurethane without the volatility drawback.
Red phosphorus flame retardants
The term red-phosphorus (P-red) is used to describe one of the allotropic forms of phosphorus. It is obtained by heating white phosphorus (P-w) at a temperature close to 300°C in the absence of oxygen. The color ranges from orange to dark violet depending on the :
- molecular weight
- particle size
- impurities
P-red is an amorphous inorganic polymer. However, X-rays have established the existence of several crystalline forms, normally present to a limited extent (< 10% w). It is well known that P-red is active as a single additive in nitrogen and/or oxygen-containing polymers such as:
| Thermoplastics |
Thermosets |
Natural fibers |
|
Polyamides |
Polyurethanes
|
Cellulose
|
| Polyesters
|
Epoxies
|
Cotton
|
|
Polycarbonates |
Melamine formaldehyde
|
| Ethylene-vinyl acetate |
Polyisocyanates
|
While it has to be applied with spumific and carbonific agents and/or with inorganic hydroxides in polyolefins, styrenics, rubbers, a.s.o. P-red is the most concentrated source of phosphorus. Thus, it is an effective flame-retardant additive at a concentration ranging from 2% to 10% wt based on polymer.
Red phosphorus flame retardants are generally applied to meet high-demanding flammability requirements. They do not form toxic smoke. They show good electrical (i.e., high CTI value) and mechanical characteristics. Its application is excluded for color reasons from white or very light-colored final articles. But, they are applied for black to medium-gray articles.
Red phosphorus-based flame retardants have high thermal stability. This allows the product to overcome drastic extrusion temperatures (up to 320°C) without:
- decomposing
- releasing dangerous substances
- producing carbonaceous residues
- causing corrosion to the extrusion equipment's
Red phosphorus flame retardants - Mode of action
The most accepted mechanism of red phosphorus FRs is based on the activity of the product in intumescent systems. Following this mechanism P-red is regarded as an acid source that:
- is mainly active in the solid phase,
- extracts oxygen and/or water from the polymers producing phosphorus acid derivatives. This undergoes dehydration at high temperatures, and
- catalyzes char formation.
This mechanism is due to the following facts:
- P-red is especially active as a sole additive in oxygen and/or nitrogen-containing polymers
- It needs co-agents in all oxygen-lacking polymers
- No massive content of phosphorus moieties is generally detected in the smoke during pyrolysis
- The LOI index of polymer articles is not very much affected by the presence of P-red
The formation of P radicals occurs during pyrolysis and combustion of P-red-containing polymers. This has been proven, by EPR measurements, in nylons. These radicals are assumed to react either with:
- oxygen, by producing phosphoric structures, or
- polymers, by acting as pro-degradant, thus promoting the dripping.
The above-mentioned mechanisms show that the product is active in the solid phase. It is also suggested that P-red can operate in the gas phase as flame poisoning is likely in volatile phosphorus compounds. According to this mechanism, P-red could generate volatile phosphorus moieties. For example, P2, PO, PO2, HPO. They are in a position to scavenge H radicals.
Discover phosphorous-based flame retardants available in our database that show the following features.
Metal hydroxide flame retardants
Metal hydroxides are the most commonly used family of halogen-free flame retardants. These mineral compounds are used in:
- polyolefins,
- thermoplastic elastomer (TPE),
- polyvinylchloride (PVC),
- rubbers,
- thermosets, and
- some engineering polymers (like polyamide).
They provide flame-retardant formulations that meet appropriate standards for many applications. Such formulations produce combustion products with low opacity, low toxicity, and minimal corrosivity. Compounding of inorganic hydroxides offers a cost-effective means to achieve low-smoke flame-retardant formulations.
In addition, inorganic hydroxides are easily handled and relatively non-toxic. Several inorganic hydroxides are replacing halogenated and phosphorus-containing FRs. This is due to their long-term effects on the environment. For example, aluminum trihydroxide (ATH), magnesium dihydroxide (MDH), etc.

Key Benefits of Metal Hydroxide Flame Retardants
Aluminum trihydroxide (ATH)
Aluminum trihydroxide (ATH) is the largest-selling inorganic hydroxide used as a flame retardant. ATH is processed at a temperature below its decomposition point (190-230°C), depending on the particle size. It is used as a flame retardant in elastomers, thermosetting resins, and thermoplastics that are processed below 200°C.
ATH obtained from the BAYER process is a gibbsite whose particle size exceeds 50µm. It can be redissolved and precipitated to produce more highly purified grades of ATH. Improvements to this process lead to decreased:
- iron,
- silica, or
- residual solid impurities.
Ground hydrates (beige to white, soda silicates, iron impurities, 1.5 to 35µm) and finely precipitated hydrates (white, bright, pure, 0.28 to 3µm) can be distinguished. The major difference between different grades of ATH is essentially the particle size and the surface treatment.
The aim of the surface treatment is to increase one or more specific mechanical properties like elongation at break (EB).
-
Fatty acids or metal stearates are often used as surface treatment of ATH or MH. This limits the aggregate of additives and increases the EB property in cable and wire applications.
- Some silane-based surface treatments are also available with unreactive (alkyl group) and reactive (vinyl, amino, epoxy, and methacryl) substituents. The kind of reactive substituent depends on the polymer in which the flame retardant is used.
Silane surface treatments are developed to perfectly suit specific applications. This is due to their higher cost compared to fatty acids. Other surface treatments include phosphorus, titanium, and zirconium instead of silicon as the central element. Titanates and zirconates have specific applications and are generally more expensive than silanes.
Discover aluminum trihydroxide (ATH) flame retardants available in our database that show the following features.
Magnesium dihydroxide (MDH)
Magnesium dihydroxide (MDH) is a more thermally stable inorganic flame retardant. It is stable at temperatures above 300°C and are used in many elastomers and resins. These include engineering plastics and other resins that are processed at higher temperatures.
It is produced using different processes from magnesium-containing ores. For example, magnesite, dolomite, or serpentinite, and from brine and seawater. Some ores such as brucite, huntite, and hydromagnesite can be used as flame retardants themselves or converted into MDH. Three different processes used to produce MDH are:
- Seawater and brine process,
- Aman Process, and
- Magnifin® process.
MDH used as flame retardant is generally of high purity (>98.5%). It is most often obtained from seawater or brine. Although it is an ore-derived product, it can also be of high purity.
Most flame-retardant grades of MDH are white powder. They have a median particle size ranging from 0.5 to 5 µm. The surface area ranges from 7 to 15 m2/g, depending on the particle shape and size. Like ATH, MDH is used at high loading levels, usually between 50% and 70%. The small quantity of MDH used as flame-retardant comes from the higher price of MDH compared to precipitated grades of ATH.
Discover magnesium dihydroxide (MDH) flame retardants available in our database that show the following features.
Melamine flame retardants
Melamine-based flame retardants represent a small but fast-growing segment in the FRs market. These products offer particular advantages over existing flame retardants:
- Cost-effectiveness
- Low smoke density and toxicity
- Low corrosion
- Safe handling
- Environmental friendliness
In this family of non-halogenated flame retardants, three chemical groups can be distinguished:
- Pure melamine
- Melamine derivatives, i.e., salts with organic or inorganic acids. For example, boric acid, cyanuric acid, phosphoric acid or pyro/poly-phosphoric acid, and
- Melamine homologues such as melam, melem and melon
Melamine-based flame retardants show excellent flame-retardant properties and versatility in use. This is because of their ability to employ various modes of flame-retardant action.
|
FR Mechanism
|
Melamine derivatives
|
Halogen/Antimony
|
Organophosphorus
|
Metal Hydroxides
|
|
Chemical interference
|

|

|

|
|
|
Heat sink
|

|
|
|

|
|
Char formation
|

|
|

|
|
|
Intumescence
|

|
|

|
|
|
Inert gas |

|

|
|

|
| Heat transfer (dripping)
|

|
|
|
|
Currently, the main areas of application for melamine-based flame retardants are:
- flexible polyurethane foams,
- intumescent coatings,
- polyamides, and
- thermoplastic polyurethanes.
Through continued research and application development, the melamine-based flame retardants market will expand. In the near future, it will grow in the direction of polyolefins and thermoplastic polyesters.
Mechanism of action of melamine compounds
FRs function by interference with one of the three components that initiate and/or support combustion:
Melamine shows excellent flame-retardant properties. This is because of its ability to interfere with the combustion process in all stages and in many different ways.
In the initial stage, melamine can retard ignition. It causes a heat sink through endothermic dissociation. This happens in the case of a melamine salt followed by endothermic sublimation of the melamine itself. This takes place at roughly 350°C. Another, even larger, heat sink effect is generated. This happens by the subsequent decomposition of the melamine vapors.
Melamine can be regarded as a "poor fuel" having a heat of combustion of only 40% of that of hydrocarbons. Furthermore, the nitrogen produced by combustion will act as an inert diluent. Another source of inert diluent is ammonia. It is released during the breakdown of the melamine or self-condensation of the melamine fraction which does not sublimate.
Melamine can also show a considerable contribution to the formation of a char layer in the intumescent process. The char layer acts as a barrier between oxygen and polymeric decomposition gases. Char stability is enhanced by multi-ring structures. For example, melem and melon are formed during the self-condensation of melamine. In combination with phosphorous synergists melamine can further increase char stability. This happens through the formation of nitrogen-phosphorous substances. Finally, melamine can act as a blowing agent for the char, enhancing the heat barrier functionality of the char layer.
Discover melamine-based flame retardants available in our database that show the following features.
Silicone-based Flame Retardants
Silicone-based flame retardants can produce protective surface coatings during a fire, caused by a low rate of heat release. Low levels of silicon in certain organic polymer systems have been reported to improve their LOI and UL-94 performance.
Some compounded silicon (polydimethylsiloxane-type) contains dry powders with a variety of organic plastics. In PS, an additive level, as low as 1 to 3%, reduces the rate of heat release by 30 to 50%. Similar improvements are reported in HIPS, PS-blends, PP, and EVA.
The study on silicon-modified polyurethane (PU) shows a decrease in the rate of release of these materials as compared to unmodified PU. The proposed mechanism is the following:
- While burning, formation on the material surface of a silicon dioxide layer can act as a thermal insulator.
- This prevents the feedback of energy to the substrate by re-radiating the external heat flux.
New silicon-based FRs for polycarbonate (PC) and PC/ABS resins offer good mechanical properties and high flame retardancy performance. These include strength, molding, and UL-94, 1/16 inch V-0 at 10 phr.
Linear and branched chain-type silicon with (hydroxy or methoxy) or without (saturated hydrocarbons) functional reactive groups are evaluated. The silicon, which has a branch chain structure, aromatic groups in the chain, and a non-reactive terminal group is very effective. In this case, the silicon is dispersed in the PC resin, and it may move to the surface during combustion to form a highly flame-retarding barrier.
Discover silicone-based flame retardants available in our database that show the following features.
Nanomaterials as flame retardants
Expandable graphite
Expandable graphite provides good flame retardancy at low loading. It can be used in thermoplastic and thermosetting resins. Expandable graphite can be used alone for natural charring polymers (PA, PU, PVC). However, it is often combined with other flame retardants such as:
- phosphates,
- boron compounds,
- antimony trioxide, or
- magnesium hydroxide
This allows the development of a strong enough substrate to support the shield of expandable graphite. Graphite can also be used in nanocomposite PP.

Expandable Graphite is used as a Flame Retardant in Thermosets and Thermoplastics
Nanoclays
Nanoclays reduce relative heat release, promote surface char, create an anti-dripping effect, and reduce smoke generation.
- In non-halogenated formulations, nanoclays allow a lower loading of mineral flame retardant.
- In halogenated systems, they reduce the amount of brominated or ATO flame retardant needed. This provides lower density, low blooming, and better mechanical properties.
Carbon nanotubes (CNTs)
Multi-walled carbon nanotubes (CNTs) are used commercially for their electrostatic dissipative (ESD), strength, and flame retardant properties. Some of their key properties are as follows:
- Effective at forming char,
- Retard the onset of combustion by drawing heat away,
- Increase viscosity to help prevent dripping, and
- Do not contribute to depolymerization.
CNTs are expected to find use in electronics, where they can provide both ESD and flame retardant properties.

Structure of Carbon Nanotubes
Polyhedral oligosilsesquioxane (POSS)
POSS-based hybrid polymers are completely defined molecules of nanoscale dimensions. They can be functionalized with reactive groups suitable for the synthesis of new organic-inorganic hybrids. POSS has been successfully incorporated into common polymers via copolymerization, grafting, or blending.
The synthesis of POSS cages, monomers containing POSS cages, POSS-dendrimer cores, POSS-containing polymers (nano building blocks), and POSS nanocomposites leads to specific properties like:
- mechanical,
- thermal,
- flame-retardant, and
- viscoelastic
They are used commercially as flame-retardant aids in phenolics, as well as in PPE and COC. A key advantage of POSS is its action as an intumescent synergist and as a dispersion aid for halogen-free flame retardants (HFFR), This may allow higher levels of HFFR by improving flow. For example, a lithiated POSS aids dispersion, provides thick intumescent char, and mitigates the loss of mechanical properties compared to using phosphate FRs alone in thermosets such as vinyl esters.
 Silsesquioxane Cage Structure
Silsesquioxane Cage Structure
Polymer-clay nanocomposites
Polymer-clay nanocomposites are hybrid organic polymer inorganic layered materials. They have unique flammability properties when compared to conventional filled polymers. Polyamide-6, polystyrene, and polypropylene are some polymers used in combination with clays.
Hyperbranched polymers
Hyperbranched polymers (HBP) with their numerous, ordered branches, open the way to hyperfunctionalized additives. They can be prepared from "branched" monomers of the general type AxBy, where:
- A and B represent functional groups that can react with each other (i.e., A + B → -A-B-) but not with themselves (i.e., A cannot react with A and B cannot react with B).
- x and y are integers (x is equal or larger than 1 and y is equal or larger than 2).
For the simplest case of AB2 monomers, the obtained polymer is represented as shown in the below figure. The figure takes into account the benefits of HBP and fire-retardant additives. It suggests some possible routes toward new fire-retardant additives.
 Fire Retardant Hyperbranched polymers
Fire Retardant Hyperbranched polymers
Dendrimers is a special form of HBP that offers additional opportunities. It starts from a single focal point or core. Each of its branches divides into two (or more) other branches down to the terminal functionalized ends. They can host metal atoms to give metallodendrimers. The metal can be situated in the repeat unit, the core, or at the end groups. Metal hosting expands the properties of metallodendrimers.
Due to their architecture, dendritic polymers can be active at low levels compared to linear polymers. The following figure compares HBP and dendrimer structures with a conventional linear macromolecule having some short branches.
 Structure of Dendrimers (L), Hyperbranched Polymers, AB2 Monomer (R) and Linear Macromolecule (Below)
Structure of Dendrimers (L), Hyperbranched Polymers, AB2 Monomer (R) and Linear Macromolecule (Below)
|
Hyperbranched Polymers
|
Dendrimers |
- A high degree of branching
- Multitude of reactive or non-reactive end-groups
- Numerous end functions
- A broad molecular size distribution
- A lower cost than dendrimers
|
- A high degree of branching with a better ordered structure leading to highly precise architectures
- A narrower molecular size distribution
- A size in the nanoscale domain
- A compact hydrodynamic volume and a Newtonian flow easing processing
- A better suitability to host various entities
- A higher cost only justified for very specific applications
- Do not contain molecular core
- Less defined intramolecular cargo space
|
Comparison of HBP with Dendrimers
Perstorp markets Boltorn™ products are produced using polyalcohol cores, hydroxy acids, and technology based on captive materials. The dendritic structures are formed by polymerization of the particular core and 2,2-dimethylol propionic acid (Bis-MPA). The obtained base products are hydroxyl-functional dendritic polyesters. They are fully aliphatic and consist only of tertiary ester bonds. They are claimed to provide excellent thermal and chemical resistance.
A wide variety of different HBPs include polyamides, polyamidoamines, polyureas, polyurethanes, polyesters, polycarbosilanes, polycarbosiloxanes, polycarbosilazanes, perfluorinated derivatives of many of the previous polymers, etc. These polymers are suited for specialty coating applications including plastic additives.
Key Considerations while Selecting the Right Flame Retardant Type
Key Considerations while Selecting the Right Flame Retardant Type
Flame retardant selection depends on:
- the type of application,
- the specific flame retardant standards, and
- the regulations to be met.
There are a number of other issues that must be considered when selecting the best FR system for a given use.
Selection criteria for brominated flame retardants
The following are the factors that may impact brominated flame-retardant selection.
-
Bromine type and content
To be effective, the selected brominated flame retardant must decompose when the polymer burns. But, it should remain stable during polymer processing. This in turn dictates the type of bromine in the FR. It must also have enough bromine content to get the required FR performance. It should not adversely affect physical properties and system costs due to high loadings.
-
Thermal stability
The selected brominated flame retardant must remain stable during compounding and injection molding. Decomposition during these steps can lead to color formation, degradation of the polymer, and equipment corrosion. Hence, selection of the correct FR along with any heat stabilizers and synergists that may be required is extremely important.
-
Aging characteristics
The resin system may have to withstand various factors responsible for premature degradation of properties and color formation. Several factors dictate the best flame retardant to be used in a specific system along with any stabilizers required. These factors include UV stability, thermal stability, and migration.
-
Processing characteristics
Depending on the processing temperature certain FRs are melt blendable while others act as fillers. This can affect the processing and final physical properties.
-
Standard to be met
Flame retardants' selection will depend heavily on the resin system that has been chosen and the standards to be met.
-
Cost in use
The overall cost of the entire package needs to be taken into account. This is not just a function of the cost of the BFRs but its required loading. It also depends on other additives that are required to be used with BFR to get a viable system.
-
Environmental concerns
The use of brominated flame retardant induces specific environmental constraints. One of the key topics is to reduce toxic hazards at each step of your production process (from manufacturing to end-use and disposal).
-
Non-blooming
Blooming is a very slow process where the flame retardant migrates to the surface of the plastic. This results in a hazy surface, which often has a bronze-like appearance. This effect is particularly undesirable for parts that also have an aesthetic function. For example, enclosures and housings. For this reason, blooming is an important criterion to consider for some applications. Generally, blooming depends on the compatibility of the FR with the polymer additive as well as the FR's molecular weight. The higher the compatibility and the molecular weight, the lower the blooming.
 Non-blooming (L), Surface Hazing (R)
Non-blooming (L), Surface Hazing (R)
-
UV stability
In many applications, the flame-retardant resin may have to withstand various conditions. These can cause premature degradation of properties and discoloration. Thus, the right BFR selection is critical for UV-stable applications and outdoor applications.
Selection criteria for chlorinated flame retardants
There are several parameters to consider while selecting a chlorinated compound include:
- Chlorine content
- Thermal stability
- Volatility, and
- Physical form
Selection criteria for organophosphorus flame retardants
-
Viscosity
The addition of phosphorus flame retardant into a PU foam formulation often has an influence on its viscosity. The viscosity of the phosphorus FR influences the:
- Processability: In most cases, low viscosity is needed
- Foaming process: Foaming is a complex process. The rheology of the material will influence the cells' size distribution and foam density
- Foam performance
-
Fogging & VOC
Fogging is the condensation of volatile substances from various materials. These materials are used in car interiors on colder surfaces. This particularly happens on the windscreen and leads to clouding on the glass surface. It is well known that the evaporation of plasticizers from dashboard materials contributes to fogging. But phosphorus FRs used in flexible PU foams can contribute particularly when FR is more volatile or has volatile impurities. Major automotive producers are putting lots of effort into minimizing this undesired effect. Certain solutions are available to reduce the fogging from FRs. These solutions also maintain excellent FR performance. Select 18+ phosphorous-based FRs that have low volatiles.
-
Migration
As traditional phosphorus-based FR exhibits low molecular weight, they tend to migrate out of the material with time. This can result in undesired effects such as:
- reduction of the FR performances after a few months (no compliancy)
- changes in surface properties (lower adhesion, printability, greasy touch)
To solve these issues, higher molecular weight phosphorus-based FRs have been developed.
-
Scorching
During the manufacturing of PU foam, heat generation and the presence of oxygen can lead to discoloration. It can also cause degradation (particularly in the core) which makes it unacceptable for many end uses. This phenomenon is called "SCORCH". Most of the time, scorch can be minimized by the addition of specific antioxidants. But, the addition of phosphorus-based FR (such as chlorophosphates) might have an influence on scorching. This is based on the concentration and nature of the FR used.
-
Foam density
As opposed to rigid PUR foams, flexible PUR foams are based on open cells allowing an easy circulation of air. As the surface of contact between air and material increases with decreasing density, the density of the PU foam will have a strong influence on the concentration of phosphorus FR needed to pass a specific FR standard.

For densities higher than 40 kg/m3, 0 to 10 phr of phosphorus FRs are generally needed. For densities between 18 and 25 kg/m3, 10 to 35 phr of phosphorus FR are needed. The severity of the test to pass will also influence the concentration of FRs needed. For very demanding applications, melamine is often used in combination with phosphorus FR.
Selection criteria for ATH and MDH
Key material parameters considered when selecting an ATH or MDH product for your flame retardant formulation include:
- median particle size
- particle size distribution
- surface area
- particle morphology
- surface chemistry
- color
These product characteristics (of the base material) will have a direct effect on the compounding process and the end properties of your compound.
Physical properties
| Property |
ATH |
MDH |
| Formula |
Al(OH)3 |
Mg(OH)2 |
| Water release |
35% |
31% |
| δH |
-280 cal/g |
-328 cal/g |
| Decomposition temperature |
230 - 300°C |
330 - 400°C |
| Processing temperature |
<200°C |
>200°C |
| Cost |
Lower |
Higher |
| Commercial Grades |
Select 200+ ATH grades |
Select 100+MDH grades |
Thermal stabilities
The figure below compares the thermal decomposition characteristics of ATH and MDH.
- ATH starts to decompose at about 220°C (428°F)
- MDH decomposes at about 330°C (626°F)
Thus, MDH has a higher thermal stability offering a wider window for compound processing. ATH is suitable for use in thermosets and certain PVC- and polyolefin-based plastic compounds. Here, the processing temperatures are generally below 200°C.
 Source: Huber Engineered Materials
Source: Huber Engineered Materials
MDH is preferred when formulating plastic compounds that need to be processed at temperatures near or above 220°C (428°F). For example, polypropylene and engineering thermoplastics. Using MDH for low-melting thermoplastics or elastomers can also enable higher processing temperatures and increased compounding throughput.
When heated to decomposition, both ATH and MDH release water of hydration that quenches the polymer and dilutes smoke. ATH releases about 35% of its weight in water vs. 31% for MDH. The process of endothermic decomposition also removes heat thus helping to retard combustion. MDH absorbs more heat (328 cal/g) than ATH (280 cal/g) on the same weight basis. Thus, higher thermal stability and greater heat removal capacity make MDH a very effective flame retardant.
Filler loading
ATH and MDH are efficient at loading levels in the order of 150 phr (60 wt%). Such high loading levels can reduce processability and mechanical and physical properties. For the same reason, although hydroxides are cheaper than most other FRs, the end cost is not favorable.
Using synergistic additives for hydrated fillers offers a means for lowering filler levels. Thus, it leads to limiting the drawbacks without compromising fire performance. Surface treatment of FR additives can also improve their efficiency. This is done with organo-silanes, zirconates, or titanates.
ATH and MDH have mutual synergistic effects allowing to improve fire retardant properties for the same total loading or to lower the total loading for the same fire behavior. The use of mixed-metal hydroxides as additives in combination with ATH or MDH increases FR performances.
Use of mixed-metal hydroxides
In extrusion trials, the replacement of pure MDH by various MDH/ATH mixtures allows reductions by:
- 15 to 20% of the die pressure
- 16 to 21% of the torque
The use of ATH/MDH mixtures and synergistic mineral FRs reduce the cost. Prices of additives are, in descending order:
Polypropylene > MDH > ATH > Common white fillers
Exploiting this and the synergistic effects, it is possible to converge on a suitable balance of fire performances. Fair physical and mechanical properties with a substantial cost saving in the order of 25-30% as illustrated below.
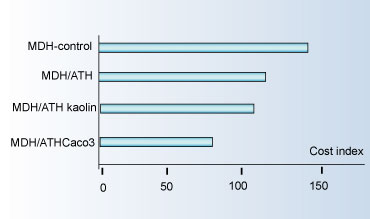 Cost-effective Benefits Using Mixed-metal Hydroxides
Cost-effective Benefits Using Mixed-metal Hydroxides
By now you must have chosen which type of flame retardant to select for your application. But is your flame retardant compatible with the plastic matrix in the formulation? Identify the best flame retardant that matches your polymer's expectations.
Find the Suitable Flame Retardant for Your Polymer
Find the Suitable Flame Retardant for Your Polymer
High Impact Polystyrene (HIPS)
- Brominated FRs are the most cost-efficient materials used for imparting flame retardancy to HIPS. Phosphorous FRs are best suited for PPO-HIPS blends.
- HIPS is used in many applications due to its excellent balance of properties and low cost. Electrical/electronics and appliances are the two most important segments requiring flame retardancy in applications where the temperature does not exceed 80°C.
Polyolefin (PO)
- Polyethylene, EVA — Wiring and cables (low and medium voltage) cable jacketing, automotive, building, and construction. ATH and MDH are the solutions of choice for demanding fire-resistant applications.
- Polypropylene — PP stands out at the edge between commodity and engineering plastics. They are commonly used in power cables, connectors, public facilities seats (stadium), fitting enclosures, and PP fibers (carpets, seats). MDH is the solution for demanding applications.
- TPO — The main application areas include roofing membranes, interior automotive applications, flexible cables, and shrinkable film.
Brominated FRs are the most cost-efficient materials for imparting flame retardancy in PE and PP.
Polyamide (PA)
- Selecting the right brominated FR for connectors is essential. MDH flame retardant allows processors to produce flame retarded PA without halogen- or phosphorous-containing compounds.
- PA applications requiring flame retardancy are components and enclosures for electrical and electronic applications.
Polybutylene Terephthalate (PBT)
- Selecting the right brominated FR for connectors is important to meet material specifications and processability (thin wall) with the lowest cost possible.
Acrylonitrile Butadiene Styrene (ABS)
- The best brominated FR for ABS depends strongly on the requirements of the final application. Phosphorus FRs are best suited for PC-ABS blends.
- Acrylonitrile contributes chemical resistance and heat stability; butadiene delivers toughness and impact strength; and the styrene component provides ABS with rigidity and processability.
Polyurethane Foams
- To achieve the desired level of performance, reactive brominated FRs are recommended. The selection of the right phosphorus FR has a major impact on the final performance.
- Rigid PU foams requiring flame retardancy are essentially used for insulation in building and construction (roofing, wall sheeting), and refrigeration.
Polyvinylchloride (PVC)
- MDH or ATH can be added as FRs to achieve desired properties.
- PVC belongs to the group of less flammable plastics, however, plasticizer addition leads to a dramatic increase in flammability and smoke density.
Natural and Synthetic Rubbers
- Flame retardancy of cross-linked elastomers using MDH or ATH has been state-of-the-art for many years.
- Typical applications include seals, gaskets, conveyor belts, cables, profiles, foams, or protective coverings.
Discover other polymers available in our database that are compatible with flame retardants.
Sustainable and Environment friendly Flame Retardants
Sustainable and Environment friendly Flame Retardants
BFR-enhanced plastics in sustainable waste management
Plastics containing BFRs have proven to be fully compatible with all methods of waste management. This is especially true for recycling and recovery. Certain plastics/BFR combinations are already being specified by leading manufacturers of photocopiers. This is in part because of their excellent stability in the recycling process.
Recycling is already taking place with 30% of some new copiers containing recycled plastic with brominated flame retardants. A recent study concluded that ABS plastic containing a BFR was superior to other plastics in terms of recyclability. They could be recycled five times. This is in full compliance with the strictest environmental and fire safety requirements.
The Swedish company, Boliden, has developed a recycling process. This is for electrical and electronic equipment waste. This is in compliance with Swedish regulation, whereby the metals are recycled. The plastics provide some of the energy in the smelting process. Brominated flame retardant-containing plastics have been tested in this process. They fully meet the smelter's requirements.
Thus, the presence of plastics containing BFRs in the waste stream provides producers with a wide variety of feasible options. These are environmentally and economically suitable for waste recovery and recycling.
Are you ready to choose the right flame retardant? Click on the link below to make your flame retardants selection easy.
