Chemistry of Silanes
Chemistry of Silanes
Silane is a molecule containing a central silicon atom bonded to two types of groups: Alkoxy groups and organo-functional groups. The generic structure of silane is mentioned below:
Y-R-Si-X3
where,
- X is a hydrolyzable alkoxy group (methoxy, ethoxy, or acetoxy) and
- Y an organofunctional group (amino-, vinyl-, epoxy-, methacryl- etc.) attached to the silicon by an alkyl bridge, R
These two types of groups exhibit different reactivity and allow sequential reactions.
Silanes as Dispersing Agents
Silanes as Dispersing Agents
Dispersing agents are used to facilitate and stabilize the dispersion of solid compounding materials such as fillers or pigments in a polymeric (or a liquid resin) matrix. Better dispersion leads to better processability and improved material properties!
Silane is one such agent that helps in easier processing and/or better product performances along with cost advantages. Get an overview of how silanes can improve the dispersibility of pigments & fillers in plastics and how they can help in better material processability and performances.

Dispersing Mechanism
During silane treatment of a filler or pigment, a reaction takes place between the functional groups of the filler or pigment (such as OH groups) and the alkoxy groups of the silane to
create a silane functionalized surface.
The surface of the filler can be functionalized to improve compatibility with the polymer matrix via specific interactions or chemical reactions between the polymer and silane organofunctional group. The functionality of the silane should be chosen to match the polymer matrix.
Silane treatment also creates a "protective layer" preventing re-agglomeration of the particles. The figure below shows the treatment of mineral surface by organosilane.
 Treatment of a Mineral Surface by an Organosilane
Treatment of a Mineral Surface by an Organosilane
Source: www.azom.com
Silane Dispersing Agents – Benefits
Silane Dispersing Agents – Benefits
The use of a silane dispersing agent in a filled thermoplastic, rubber, or thermoset formulation results in several benefits which ultimately translate into easier processing and/or better product performances. During masterbatch production, the use of Silane treated
pigments allows higher pigment loadings or higher production rates.
The use of silane as dispersing agent provides significant performance and cost advantages.
Learn the various benefits that silane dispersing agents provide to material or click on the specific benefit to address a specific issue:
- Better Dispersion and Wet-out
- Lower Viscosity of Filled Liquid Resins
- Reduced Cure Inhibition of Resin
- Improved Electrical Properties
 Benefits of Silane Dispersing Agents
Benefits of Silane Dispersing Agents
#1. Better Dispersion and Wet-out with Silane
Using silane dispersing agents leads to a significant improvement of filler and pigment dispersion in a polymer system. The polymers can be thermoset, thermoplastic, or even rubber elastomer networks.
This improvement is due to surface modification of the filler making it more compatible with the polymeric matrix and improving the wettability of the filler. Silanes such as
XIAMETER™ Z-6070 Silane also create a "protective layer" minimizing re-agglomeration of the particles and sealing off the effects of the surface on resin cure and electrical properties.
 Comparison between Filler treated silane and Untreated Silane
Comparison between Filler treated silane and Untreated Silane
For thermoplastics systems, an improved dispersion results in:
- Easier filler or pigment incorporation (higher loading, wider processing window)
- Lower material viscosity
- Lower surface defects
- Better mechanical properties
- Better opacity for pigments (ex: Titanium Dioxide, TiO2)
For liquid resin systems, improved dispersion often results in less air occlusion and reduced slurry viscosity, allowing easier flow during molding and the possibility of using increased proportions of inexpensive filler.
#2. Lower Viscosity with Silane
Introducing filler into a molten polymer tends to increase the melt viscosity of the mixture. The viscosity increase depends on numerous parameters like:
- Viscosity of the molten polymer
- Filler concentration
- Quality of the wetting between the polymer and the filler
- Particle size distribution
Treating filler particles with silanes enables better wetting of the filler by the polymer, helps the filler remain well dispersed, and gives a lower viscosity compound than with untreated fillers. This leads to easier processability, higher throughput, better surface quality, and higher filler loading in masterbatches.
Silane treated fillers and pigments allow higher throughput,
better surface quality and higher loadings
The figure below shows the influence of silane treatment on the melt temperature and torque percent during the production of a PE/TiO2 masterbatch at 80 weight percent TiO2.
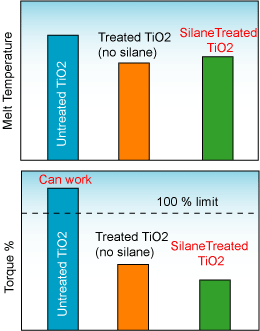 Silane Treated TiO2 allowing low torque and higher throughput
Silane Treated TiO2 allowing low torque and higher throughput
#3. Reduced Cure Inhibition
Fillers are known to have varying degrees of effect on the cure systems of thermoset resins that can inhibit their cure. Using silanes as dispersing agents can lead to reduced cure inhibition.
Silane treatment of fillers in both polyesters and epoxies often overcomes cure inhibition as measured by cure exotherms (see the figure below). Silanes that were generally the most effective dispersing agents often enabled the highest exotherms.
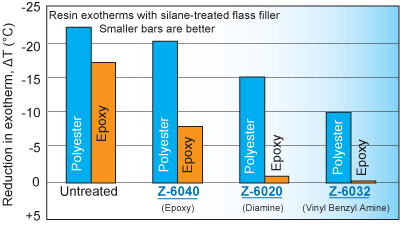 Silanes that allowed maximum exotherms were generally the most effective dispersing agents
Silanes that allowed maximum exotherms were generally the most effective dispersing agents
#4. Improved Electrical Properties
The ability of silane dispersing agents to impart improved electrical properties is shown in the table below with an epoxy resin reinforced with quartz filler.
The table below shows an improvement in electrical properties with silane dispersing agents in quartz-reinforced epoxy resins:
|
System
|
Dielectric Constant
|
Dissipation Factor
|
|
Dry
|
Wet*
|
Dry
|
Wet*
|
| Unfilled resin |
3.44
|
3.43
|
0.007
|
0.005
|
| Quartz, no silane |
3.39
|
14.60
|
0.017
|
0.305
|
| Quartz, XIAMETER™ OFS-6040 Silane |
3.40
|
3.44
|
0.016
|
0.024
|
| Quartz, XIAMETER™ OFS-6011 Silane |
3.46
|
3.47
|
0.013
|
0.023
|
* After 72 hours immersion in boiling water
|
Without filler, the epoxy resin showed good electrical properties, dielectric constant, and dissipation factor, even after aging for 72 hours in boiling water. However, once quartz filler was added, the hydrophilic surface of the quartz led to a severe loss of electrical properties during the water boil test. With either XIAMETER™ OFS-6040 Silane, XIAMETER™ OFS-6011 Silane, or XIAMETER™ OFS-6070 Silane, the quartz-filled composite exhibits dramatic retention of electrical properties.
Filler Treatment with Silane Dispersing Agents
Filler Treatment with Silane Dispersing Agents
Mineral fillers have become increasingly important additives and modifiers for organic polymer.
Silanes are a natural fit to treat the surface of the mineral to make the mineral more dispersible in the polymer.
The different applications of silane dispersing agents in mineral treatment:
The table below lists some benefits of silane treated filler:
| Benefits of Silane Treated Filler |
|
Processing benefits
Better dispersion leads to:
- Higher filler loading
- Lower die build-up
- Higher production rates
- Wider processing window
|
Products benefits
Better dispersion leads to:
- Better color stability
- Higher gloss and optical quality
- Better mechanical properties
|
TiO2 Treatment
TiO2 is the most commonly used white pigment for plastics. It exhibits excellent whiteness, excellent opacity, and good UV resistance. Most compounders and master batchers process TiO2, and all require the following attributes to maintain high-quality standards and competitive prices.
The table below shows the key requirements for materials or masterbatches containing TiO2:
|
Process requirements
|
Product requirements
|
- Low torque, low pressure (high rates)
- Excellent dispersion
- High filler loadings for masterbatch
- Lacing resistance
- Low abrasion
- Low die build-up
|
- Good color stability and whiteness
- High opacity
- Cost-effectiveness
- Excellent mechanical retention
- Good surface quality (for films, sheets...)
|
To achieve this, TiO2
is treated with silane dispersing agents such as XIAMETER™ OFS-6070 Silane.
The use of silane treated TiO2 improves TiO2 dispersibiliy
as well as the performance of the TiO2-filled plastic.
The figure below shows how a silane treatment reduces the melt temperature and the torque required in the compounding machine during the production of a PE/TiO2 masterbatch at 80% TiO2 loading.
 Reduction of the melt temperature and torque of a compounding machine with the addition of silanes
Reduction of the melt temperature and torque of a compounding machine with the addition of silanes

Talc Treatment with Silane
Talc is a platy filler commonly used as a reinforcer in polyolefins (PE, PP, EVA), styrenics and occasionally in engineering polymers. Talc is used to increase HDT and stiffness and can reduce creep, shrinkage, and coefficient of linear thermal expansion (CLTE).
To achieve this, talc is treated with silane dispersing agents such as XIAMETER™ OFS-6070 Silane.
The use of silane treated talc improves talc dispersibility
as well as the performance of the talc-filled plastic.
Wollastonite Treatment with Silane
Wollastonite is a white acicular filler imparting good dimensional stability, good scratch resistance, and excellent stiffness to thermoplastics such as PP or PA. Main applications are automotive parts such as trims, bumpers, or instrument panels.
Using silane dispersing agents such as XIAMETER™ OFS-6070 Silane to treat Wollastonite provides these performances.
The use of silane treated wollastonite improves wollastonite dispersibility
as well as the performance of the wollastonite-filled plastic.
Silanes as Coupling Agents
Silanes as Coupling Agents
Coupling agents are adhesion promoters that are used to provide a stable bond by reducing the interfacial tension between the fibrous or particulate inorganic component and the organic matrix polymer in reinforced and filled plastics. This improved bond results in greater composite strength and longer service life of reinforced and filled plastics.
In simple words, silane coupling agents will act as a link between an inorganic substrate (such as glass, metal, mineral) and an organic material (such as an organic polymer, coating, adhesive) to bond, or couple, the two dissimilar materials together.
 Silane Coupling Mechanism
Silane Coupling Mechanism
Coupling Mechanism
The alkoxy groups react with the surface groups of many inorganic fillers. They first react with water to produce the silane triol, releasing alcohol as a by-product. The silanol groups then condense with oxide or hydroxyl groups on the filler surface. Neighboring siloxane chains can interact further to produce a polysiloxane layer at the surface.
Coupling of a typical silane (gamma-aminopropyltrimethoxysilane) to a siliceous substrate
Silanes require active sites, preferably hydroxyl groups, on the filler surface for reaction to occur. They can therefore be used to treat all:
- Silicate-type fillers
- Inorganic metal oxides and hydroxides
Silane Coupling Agents – Benefits
Silane Coupling Agents – Benefits
Improved Flexural Strength
Silane coupling agents provide the benefit of imparting good mechanical properties such as tensile, flexural and compressive strengths. The figure below shows the improvement of flexural strength of glass fiber reinforced epoxy resins obtained by using silane coupling agents.
 Improvement of flexural strength with silanes coupling agents
Improvement of flexural strength with silanes coupling agents
Higher Modulus
Silane coupling agents when used after filling rubber offer another benefit of imparting a higher modulus. This improvement in clay-filled rubber is shown in the table below.
| Rubber Type |
Clay Treatment |
300% Modulus (psi) |
| Natural rubber |
None |
1040 |
| 1% XIAMETER™ OFS-6020 Silane |
1655 |
| SBR rubber |
None |
285 |
| 1% XIAMETER™ OFS-6020 Silane |
400 |
| Nitrile |
None |
1230 |
| 1% XIAMETER™ OFS-6020 Silane |
2125 |
Improvement of modulus with silanes coupling agents in clay-filled rubber
Improved Filler and Pigment Dispersion
Using silane coupling agents leads to a significant improvement of filler and pigment
dispersion in resin (see the figure below). This improvement results from
displacement or modification of the moisture layer, giving reduced clumping of particles
and improved wettability by the polymer.
 Improvement of dispersion with silanes coupling agents
Improvement of dispersion with silanes coupling agents
This improved dispersion often results in less air occlusion, giving fewer voids
and reduced slurry viscosity. Easier flow in molding or
increased proportions of inexpensive filler, or both, are possible.
Higher Production Rate
Due to the lower viscosity of the composite possible by adding
silane coupling agents (table below), improved processability in compounding and injection molding
is usually observed, leading to a higher production rate.
Viscosity of polyester composites with 50% silica
Less Cure Inhibition
Fillers are known to have varying degrees of inhibition of the curing of thermoset
resins. Using silanes can cover and seal the filler surface to prevent tag interaction
of the filler with the curatives. In both polyesters and epoxies, it was observed
that silane treatment of fillers often overcomes cure inhibition as measured by
cure exotherms (see the table below). Silanes that allowed maximum exotherms were
generally the most effective coupling agents.
|
System
|
Reduction Of Cure
Exotherm ΔT (°C)
|
|
Polyester
|
Epoxy
|
|
Untreated
|
- 22
|
- 17
|
|
XIAMETER™ OFS-6040 Silane (Epoxy)
|
- 20
|
- 8
|
|
XIAMETER™ OFS-6020 Silane (Diamine)
|
- 15
|
- 1
|
|
XIAMETER™ OFS-6032 Silane (Cationic Styryl)
|
- 10
|
0
|
Resin exotherms with silane-treated filler
Filler Treatment with Silane Coupling Agents
Filler Treatment with Silane Coupling Agents
The silane treatment can improve processing, performance, and durability of a mineral, silica, glass fiber, and bead by:
| Mineral Filler & Silica Treatment |
Glass Fiber and Bead Treatment |
- Improving adhesion between the mineral and the polymer
- Improving wet-out of the mineral by the polymer
- Improving dispersion of the mineral in the polymer
- Improving electrical properties
- Increasing mechanical properties
- Reducing the viscosity of the filler/polymer mix
|
- Increased mechanical strength of the composites
- Improved electrical properties
- Improved resistance to moisture attack at the interface
- Improved wet-out of the glass fiber
- Improved fiber strand integrity, protection and handling
- Improved resistance to hot solder during fabrication
- Improved performance in cycling tests from hot to cold extremes
|
Mineral Filler Treatment
Mineral fillers have become increasingly important additives and modifiers for organic polymer. The metal hydroxyl groups on the surface of minerals are usually very hydrophobic and very incompatible with organic polymers.
Silanes are a natural fit to treat the surface of the mineral to make the mineral more compatible and dispersible in the polymer, or even make the filler into a reinforcing additive.
Silanes used as coupling agents in filler treatment are useful for many applications, some examples are HFFR wire & cable compounds, Mica-filled polypropylene and polyamide, and clays in rubber.
Silica Treatment
Silane coupling agents are often used to treat silica (both fumed and precipitated) treatment with great effectiveness in filled polymer systems. Silanes used as coupling agents in silica treatment are useful for many applications, such as green tires, shoe soles.
Glass Fiber and Bead Treatment
Silane coupling agents are a critical component of glass-reinforced polymers. The glass is very hydrophilic and attracts water to the interface. Without silane treatment on the glass surface, the bond between the glass fiber and the resin would weaken and eventually fail, making a composite essentially useless.
Silane coupling agents are used in glass treatment (fiber, bead...) for general purpose applications, such as automotive, marine, sporting goods, and construction, as well as for high-performance applications in printed circuits boards and aerospace composites.
Glass materials treated with silane coupling agents can be used either in thermosets or in thermoplastics, or any other desired polymer system.
Take the course by Chris DeArmitt to make sure your dispersing & coupling agents deliver their best by meeting yield strength, processability cost…targets without adding additives or fillers in excess in your filled plastics (i.e. avoid extra cost and cascading issues).

Silanes as Crosslinking Agents for Polyethylene
Silanes as Crosslinking Agents for Polyethylene
Crosslinking is a type of polymerization reaction that branches out from the main molecular chain to form a network of chemical links. Hence, cross-linking agents are added to resins to enable this process.
Silanes when crosslinked with PE are called PEX or XLPE. For high-performance polyethylene applications, requiring higher temperature, creep, abrasion and chemical resistances, crosslinking is a must. Of all the crosslinking technologies, Silane is the one which exhibits:
- Greatest process flexibility (by providing the possibility to trigger the crosslinking after extrusion)
- Superior mechanical performance
As opposed to other processes, Silane Crosslinking technology is easy to implement and does not require special processing equipment. Crosslinking with Vinylsilanes is flexible, easy and cost-effective.
Crosslinking Mechanism
Silane technology consists of two steps:
- Step 1: Incorporation of the silane into the polymer, either by grafting of vinylsilane onto the polymer backbone or by copolymerization of vinylsilane with ethylene in the polymerization reactor.
Grafting of Vinylsilanes onto Polyethylene
- Step 2: Crosslinking in the presence of water, generally catalyzed by tin compounds or other suitable catalysts. This second step can be controlled and made during or after the extrusion process. This is the difference between a One-Step and a Two-Step Process.
Moisture Crosslinking of Silane-Grafted Polyethylene
The use of silanes results in a more flexible and more economical process for crosslinking. Silane crosslinked polyolefins are linked through an Si-O-Si moiety instead of the C-to-C bond created via peroxide or radiation cure. Siloxane bridges are less rigid than C-to-C bonds and give flexibility to the crosslinked polymer, as shown in the figure below.
|
C-to-C bond:
rigid
|
Si-O-Si bond: flexible
|
|
|
|
Silane Crosslinking Agents – Processing Benefits
Silane Crosslinking Agents – Processing Benefits
Silane Crosslinking technology is by far the most flexible and easiest to implement. The two-step process (SIOPLAS) can often fit in existing PE extrusion equipment, requiring no further investment.
The table below compares Silane technology against Beta irradiation and a traditional free radical process (peroxide + co-monomer). It shows that Silane exhibits a unique set of cost-effective processing benefits:
| Process benefits |
Silanes
|
Peroxide (+co-monomer*)
|
Beta Irradiation
|
| Trigger Crosslinking at a desired time |
Yes
|
No
|
Yes(*)
|
| Special equipment required |
No
|
Yes
|
Yes(*)
|
| Reactivity adjustment |
Yes
|
No
|
No
|
| Sequential steps |
Yes
|
No
|
Yes(*)
|
| High Extrusion Speed |
Yes
|
No
|
Yes/No
|
| Dry process (no water) |
No
|
Yes
|
Yes
|
(*) Beta irradiation equipment remains costly. This also may require the uncured extrusion to be transported to an off-site facility for curing. Cost for investment, shipment and handling must be evaluated carefully.
|
Trigger Crosslinking at a Desired Time with Silane
One unique benefit offered by Silane crosslinking technology is its ability to trigger the crosslinking at the desired time and particularly after the extrusion of the product. With Silane technology, crosslinking can be triggered at the desired time.
Crosslinking of polyethylene is done by grafting a trialkoxysilyl group onto the PE polymer chain. Once this is done, the combination of a tin catalyst and moisture will cause the alkoxysilyl groups to react together to form a crosslink between the polymer chains. There are 2 main processes used to make PEX:
- Monosil (One-step process) – The Monosil technique introduces in a single step a mixture of Vinylsilane-peroxide-crosslinking catalyst antioxidant into polyethylene during a conventional extrusion process (such as pipe or cable). The finished product is moisture-cured (water bath or steam sauna).
Advantages of Monosil process include:
-
Cost-effective on a larger scale
- Single step-high speed
- Lowest variable cost
- Wide formulation latitude and wide customization
- No additional heat history to the PE
 Monosil (One-step process)
Monosil (One-step process)
- Sioplas (Two-step process) – In the Sioplas process, Polyethylene is first grafted in the presence of a mixture of Vinylsilane and peroxide to make a crosslinkable polyethylene. The material can be either processed directly or stored in dry conditions for up to several months.
In a separate step, the crosslinking catalyst, typically a tin derivative such as dibultinlaurate (DBTDL), and an antioxidant are mixed with polyethylene in a single or twin-screw extruder. This is the catalyst masterbatch, part B, to be used with the silane polyethylene, part A.
In a second step, grafted polyethylene is dry blended with a catalyst masterbatch (a concentrate of Tin derivative in PE), in a traditional single screw extrusion process.
The extrudate is most of the time cooled down into a water bath which provides the moisture necessary for crosslinking. The reaction is fast but the diffusion of moisture in the material is a limiting factor. For this reason, hot water bath or low-pressure steam autoclave are often used to speed up crosslinking.
Advantages of Sioplas process include:
-
Wide range of applications
- Multiple suppliers/sourcing options
- Cost-effective significant
- No investment needed (conventional equipment usable)
- Can use reinforcements
 Sioplas (Two-step process)
Sioplas (Two-step process)
Adjustment of Reactivity with Silane
Two key elements to consider during the development of a new Sioplas or Monosil
process are:
- The crosslinking kinetic which need to be adapted to residence time in the extruder,
the curing conditions, and the thickness of the parts
- The performance of the final parts depends strongly on the polyethylene grade
used and its final degree of Crosslinking.
Silane technology offers great flexibility in reactivity and
performance adjustment
As Silane crosslinking technology always requires the combination
Vinylsilane-Peroxide-Catalyst, the reactivity of the system
and the final properties of the final product can be easily adapted to any process
by adjusting the:
-
Nature and concentration of the vinylsilane (VTMOS, VTEOS or silane mixtures)
- Effect of silane structure
It is well known from silicone chemistry that the reactions which are involved in the crosslinking process become slower with the increase in the size of the alkoxy group.
VTMOS (Vinyl trimethoxysilane) reacts much faster than VTEOS (Vinyltriethoxysilanes).
The crosslinking kinetic can be then adjusted by selecting one or the other or by using mixtures at different ratios.
The figure below compares the crosslinking rates of VTMOS and VTEOS at 2 different temperatures.
Comparing crosslinking rates of VTMOS and VTEOS at 2 different temperatures
The final degree of crosslinking with these other silanes (used in the same formulation and at same conditions) may be somewhat lower versus VTMOS.
- Effect of silane concentration
The maximum concentration of VTMOS recommended is 2wt%. Of course, lower concentrations can be used but these can result in lower gel contents which might very well prove to be suitable for some applications. Concentrations higher than 2wt% do not lead to significant higher gel contents.
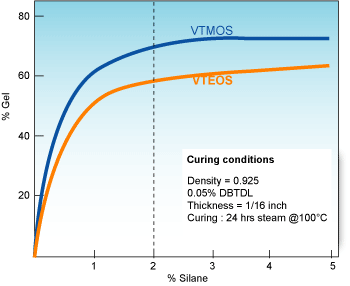 Comparing crosslinking rates of VTMOS & VTEOS at recommended concentrations
Comparing crosslinking rates of VTMOS & VTEOS at recommended concentrations
-
Nature and concentration of the organic peroxide
The peroxide to be used needs to have decomposition kinetics which is suitable for the temperatures and residence times involved in the processing of polyethylene. Generally DCP, Dicumyl peroxide or BPDIB; t-Butyl di-iso propyl benzene are recommended.
The peroxide level required will vary with individual characteristics such as :
- Melt flow index the initial polyethylene, density, polymerization process...)
- Desired melt flow index of the grafted PE
- Processing temperature during grafting
- Presence of some additives or fillers
- Nature and concentration of the condensation catalyst
DBTDL, dibutyltindilaurate is the preferred catalyst for crosslinking. Since the concentration of DBTDL affects significantly the processability of the mixture of graft copolymers, it is sometimes recommended to use it as low as 0.25wt%.
This reduction in concentration reduces the rate of crosslinking but does not affect the gel content of fully crosslinked graft copolymer
- Processing conditions and wall thickness of the extruded profile
- Process conditions – They have a strong impact on moisture crosslinking and final performance. Two key parameters are water temperature and wall thickness of the extruded profile.
- Water temperature – Increasing the temperature of the water, or the use of low-pressure steam leads to an increase in the rate of crosslinking. The figure below shows how water temperature influences the crosslinking kinetic for one specific system.
- Wall Thickness – The Crosslinking reaction is fast but the diffusion of moisture into the material is a limiting factor. For this reason, the wall thickness of the extruder parts has a strong influence on the time needed to crosslink it.
Studies have shown that the cure time is controlled by a Fick equation law. The figure below shows that as the thickness of a sample is doubled the cure time is squared.
High-Speed Line with Silane
Compared to other technologies, Silane crosslinking technology allows higher extrusion speed. As opposed to traditional crosslinking processes involving organic peroxide (eventually combined with a co-monomer), no crosslinking occurs inside the extruder even at high temperatures.
For applications requiring high extrusion speed, Silane crosslinking technology is the perfect candidate
During a traditional peroxide crosslinking process, peroxide decomposition needs to be carried out after extrusion in order to avoid the Scorch effect. Consequently, extrusion temperature needs to remain below the decomposition temperature of the peroxide. For this reason, this technology does not allow high extrusion temperatures. With a silane curing system, extrusion speed is mainly limited by the maximum die pressure and extrusion instability such as sharkskin. (see figure below)
No Special Equipment Required with Silane
SIOPLAS process implementation does not require investment
By adopting a SIOPLAS process, converters have the ability to use commercially available pre-grafted polyethylene (Silanized PE) that can be premixed with a catalyst masterbatch and color masterbatch. In this case, the process can be carried out with traditional blending and feeding equipment and with conventional single-screw extruders.

Silane Crosslinking Agents – End-product Performances
Silane Crosslinking Agents – Selection Criteria
Silane Crosslinking Agents – Selection Criteria
Even though silane technology is easy to implement, the selection of the right system needs to integrate many parameters including processing conditions and constraints, end-product performances, safety, and environmental issues, and crosslinking conditions in order to optimize cost/performances.
As always with reactive processes, the formulation to be used will not only depend on the final performances but also on the process conditions: both are strongly linked!
| Formulation parameters |
Process parameters
|
- Properties of the polymer
- PE density
- Polymerization process
- MFI
- Presence of additives and modifiers
- Desired Crosslinking degree (influence final performances)
|
- One or two step processes
- Extruder L/D
- Desired throughput
- Wall thickness of the extruded part
- Curing conditions (water bath, steam autoclave)
|
Key criteria having an influence on the selection of final crosslinking system (silane-peroxide-catalyst mixture)
Silane Ingredients for Plastics
View a wide range of silanes available today, analyze technical data of each product, get technical assistance or request samples.